

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307542 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300504 (CHEMBL574455 | N-({3-[(5S)-5-tert-butyl-1-[(4-fluo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307547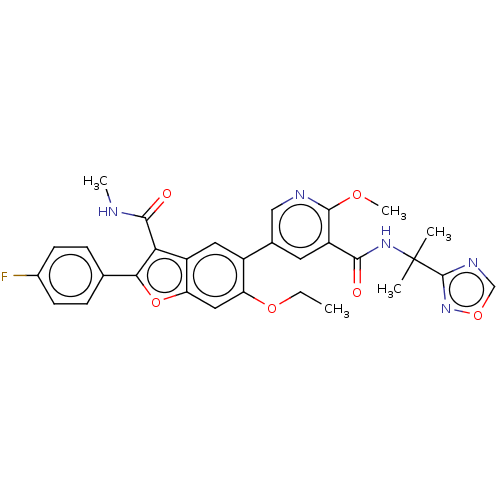 (BDBM307549 | N-(2-(1,2,4-oxadiazol-3-yl)propan-2-y...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.52 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307550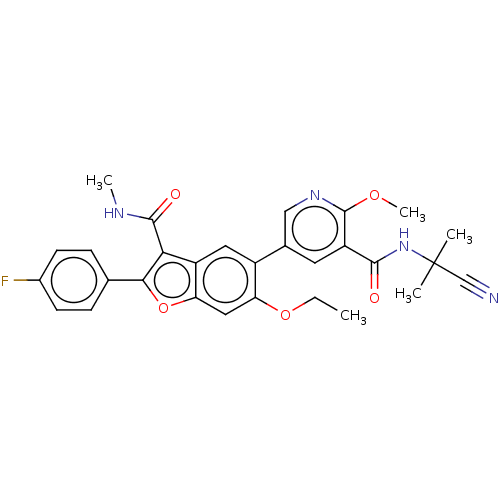 (N-(2-cyanopropan-2-yl)-5-(6-ethoxy-2-(4-fluorophen...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.84 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307545 (6-Ethoxy-2-(4-fluorophenyl)-5-(4-methoxy-3-((1-(py...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.89 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301911 (CHEMBL583269 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307561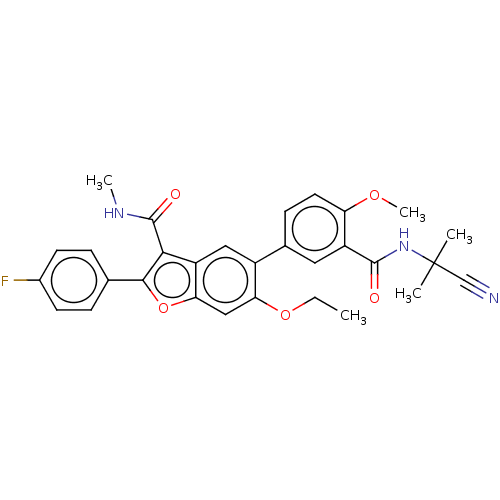 (5-(3-((2-cyanopropan-2-yl)carbamoyl)-4-(methoxy-d3...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.04 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307566 (5-(3-((2-(1,2,4-thiadiazol-3-yl)propan-2-yl)carbam...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.04 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM149222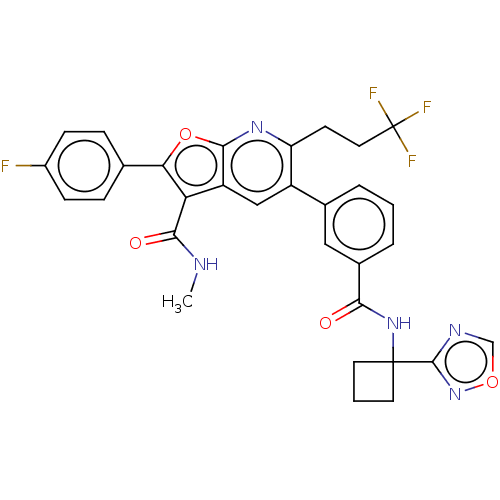 (US8962651, 4) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.05 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ... | US Patent US8962651 (2015) BindingDB Entry DOI: 10.7270/Q23R0RM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307542 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM149221 (US8962651, 1) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.32 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ... | US Patent US8962651 (2015) BindingDB Entry DOI: 10.7270/Q23R0RM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM47174 (US8962651, 17) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ... | US Patent US8962651 (2015) BindingDB Entry DOI: 10.7270/Q23R0RM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307558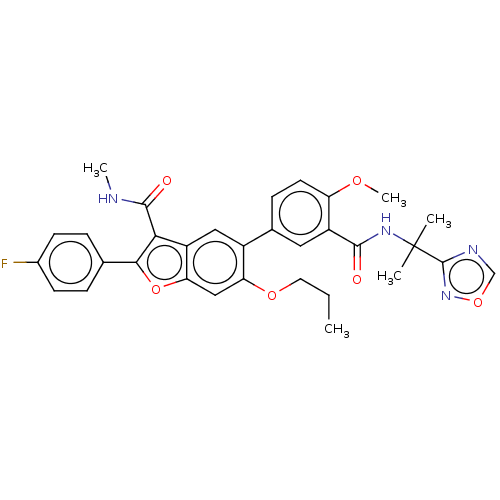 (5-(3-((2-(1,2,4-oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.48 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307548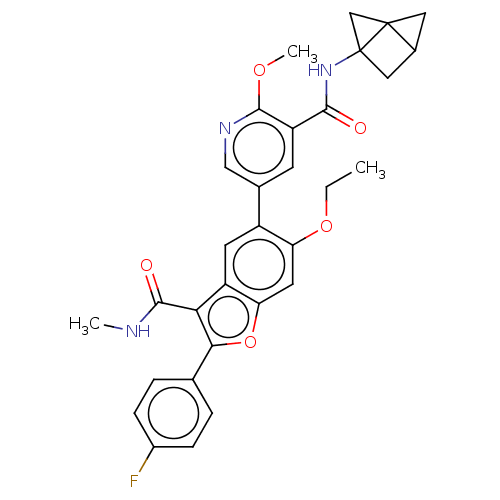 (N-(bicyclo[1.1.1]pentan-1-yl)-5-(6-ethoxy-2-(4-flu...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.48 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307546 (5-(6-Ethoxy-2-(4-fluorophenyl)-3-(methylcarbamoyl)...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.72 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307547 (BDBM307549 | N-(2-(1,2,4-oxadiazol-3-yl)propan-2-y...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307551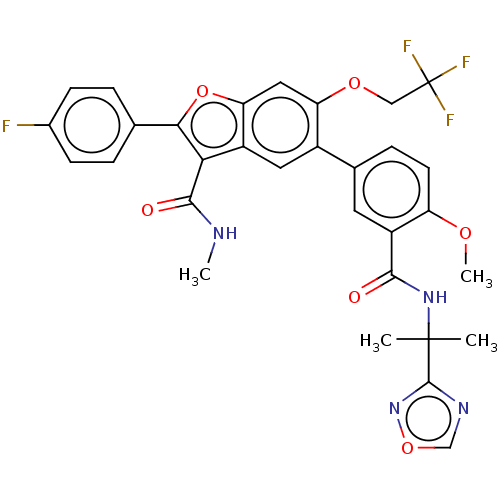 (5-(3-((2-(1,2,4-oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.83 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM149225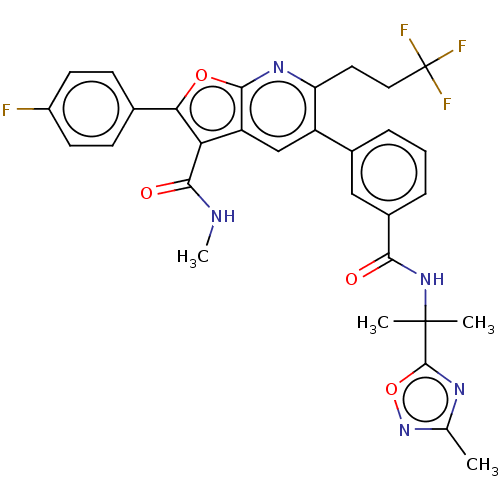 (US8962651, 9) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.87 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ... | US Patent US8962651 (2015) BindingDB Entry DOI: 10.7270/Q23R0RM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307562 (5-(2-(4-Fluorophenyl)-3-(methylcarbamoyl)-6-(2,2,2...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.95 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300502 (CHEMBL572682 | N-({3-[(5S)-5-tert-butyl-1-[(4-fluo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300497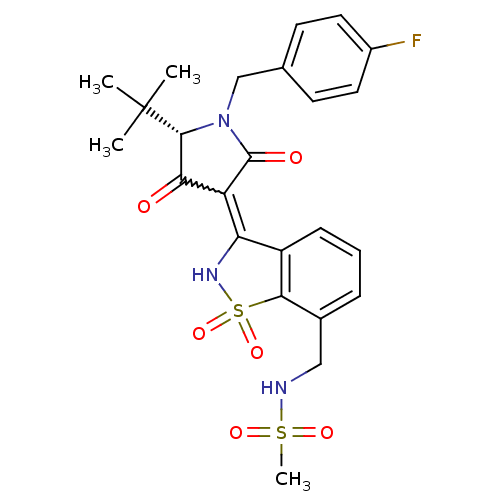 (CHEMBL578433 | N-({3-[(5S)-5-tert-butyl-1-(4-fluor...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300503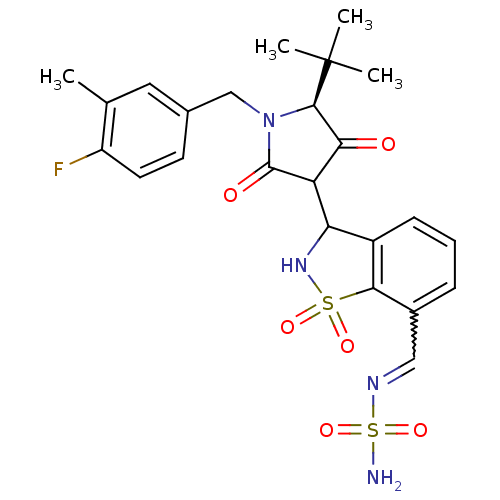 (CHEMBL582995 | N-({3-[(5S)-5-tert-butyl-1-[(4-fluo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 1b (isolate BK) (HCV)) | BDBM203919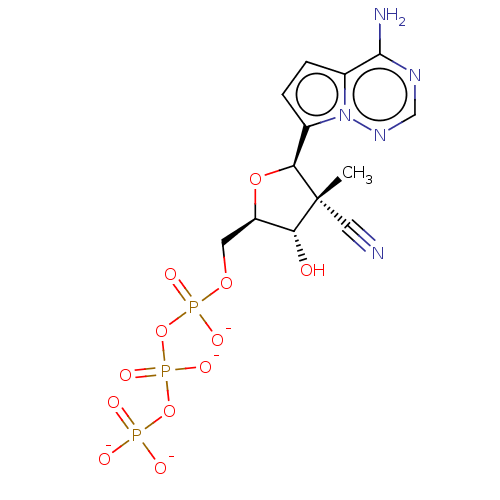 (US9242988, 1) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.3 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description To measure inhibition of the enzymatic activity of the HCV NS5B RNA-dependent RNA polymerase by the nucleoside triphosphate compounds of the present ... | US Patent US9242988 (2016) BindingDB Entry DOI: 10.7270/Q22B8WVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301902 (CHEMBL571825 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307543 (5-(3-((2-Cyanopropan-2-yl)carbamoyl)phenyl)-2-(4-f...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.02 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307539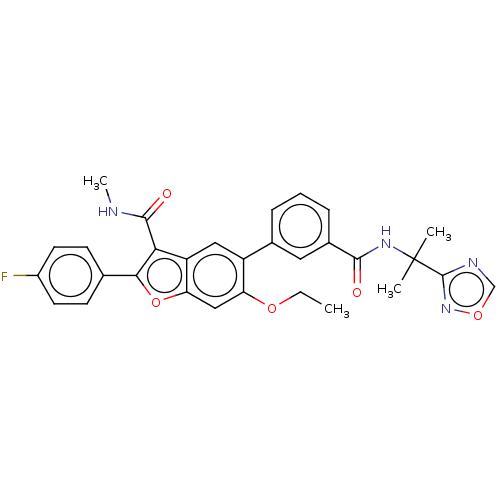 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM149227 (US8962651, 13) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.42 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ... | US Patent US8962651 (2015) BindingDB Entry DOI: 10.7270/Q23R0RM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307537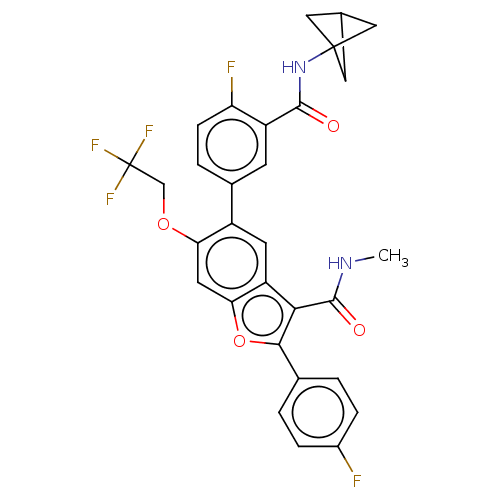 (5-(3-(Bicyclo[1.1.1]pentan-1-ylcarbamoyl)-4-fluoro...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.88 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307567 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.89 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300499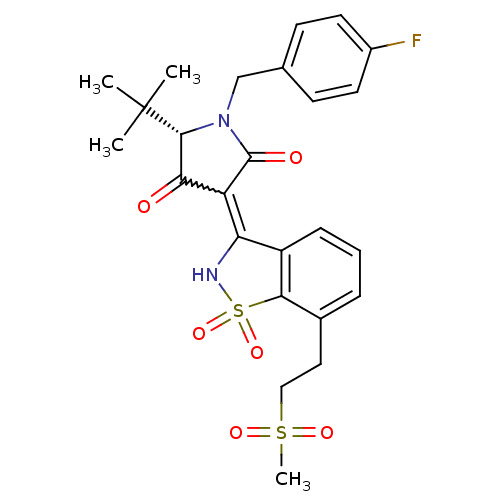 ((S)-5-tert-Butyl-1-(4-fluoro-benzyl)-4-hydroxy-3-[...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300498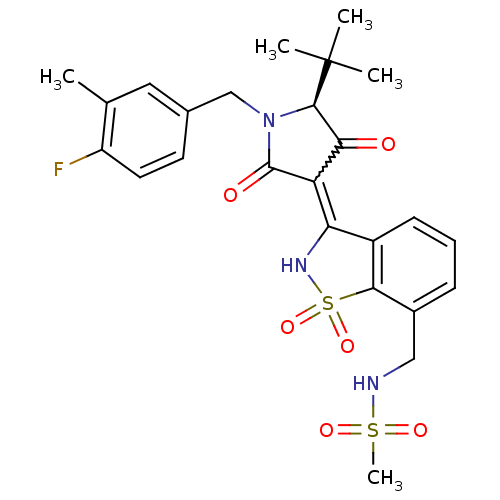 (CHEMBL575777 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301903 (CHEMBL569120 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301914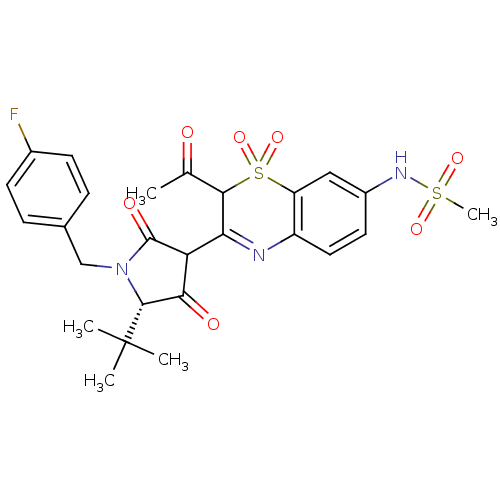 (CHEMBL570249 | N-{2-Acetyl-3-[(S)-5-tert-butyl-1-(...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM149223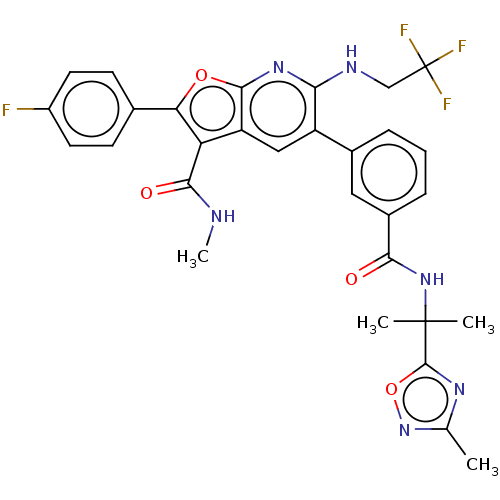 (US8962651, 7) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.13 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ... | US Patent US8962651 (2015) BindingDB Entry DOI: 10.7270/Q23R0RM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307535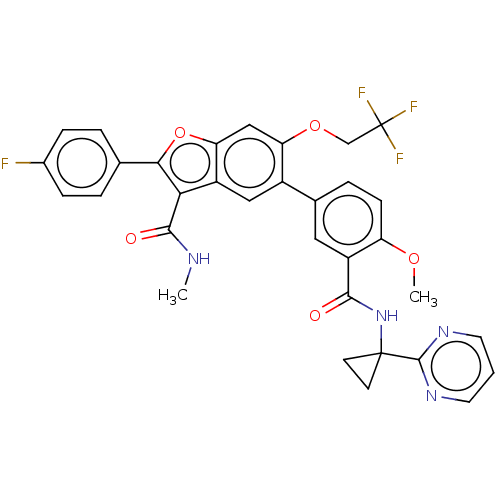 (2-(4-Fluorophenyl)-5-(4-methoxy-3-((1-(pyrimidin-2...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307552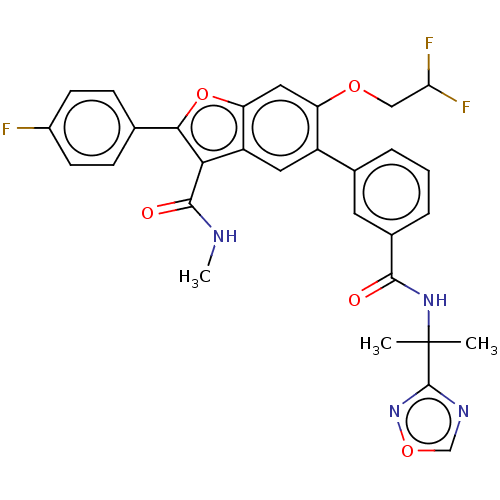 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307538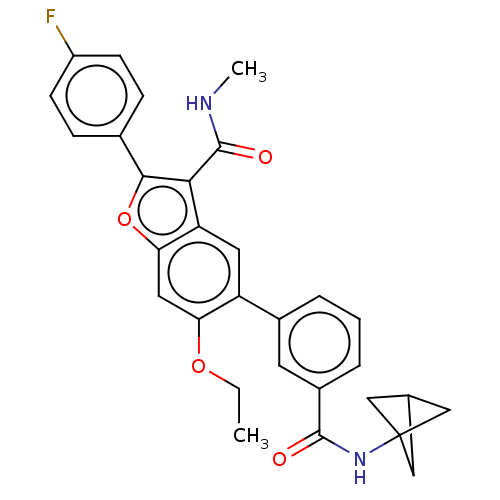 (5-(3-(Bicyclo[1.1.1]pentan-1-ylcarbamoyl)phenyl)-6...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307536 (5-(4-Fluoro-3-((1-(pyrimidin-2-yl)cyclopropyl)carb...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307554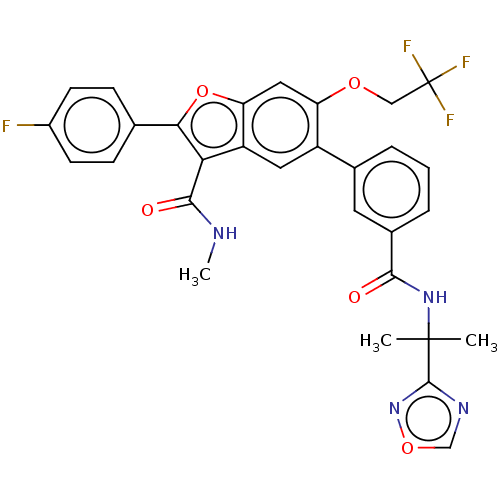 (5-(3-((2-(1,2,4-oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.49 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307553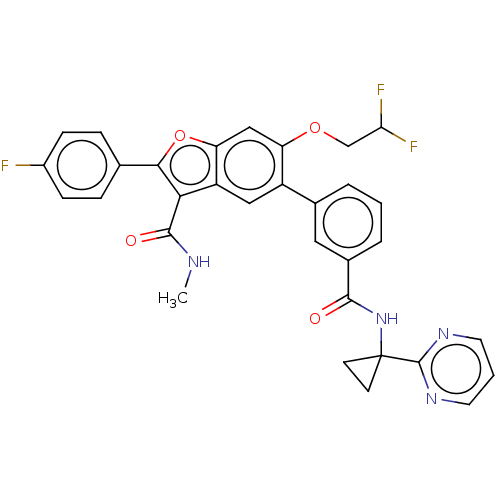 (6-(2,2-difluoroethoxy)-2-(4-fluorophenyl)-N-methyl...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.54 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM149224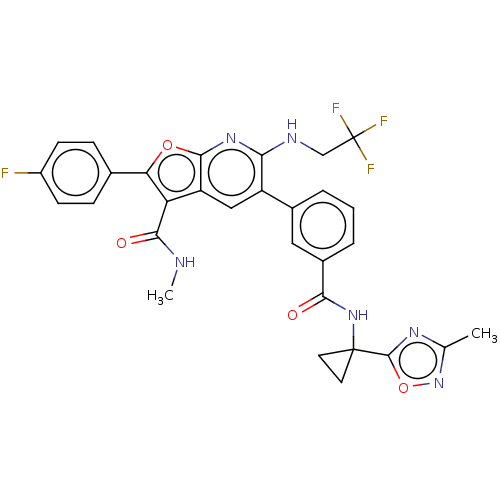 (US8962651, 8) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description An on-bead solid phase homogeneous assay was used in a 384-well format to assess NS5B inhibitors (WangY-K, Rigat K, Roberts S, and Gao M (2006) Anal ... | US Patent US8962651 (2015) BindingDB Entry DOI: 10.7270/Q23R0RM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307541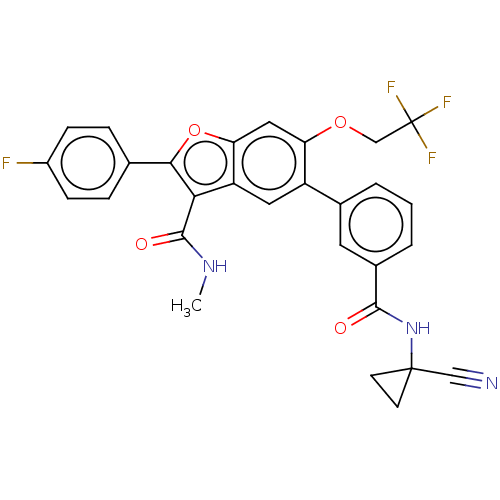 (5-(3-((1-Cyanocyclopropyl)carbamoyl)phenyl)-2-(4-f...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.97 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301901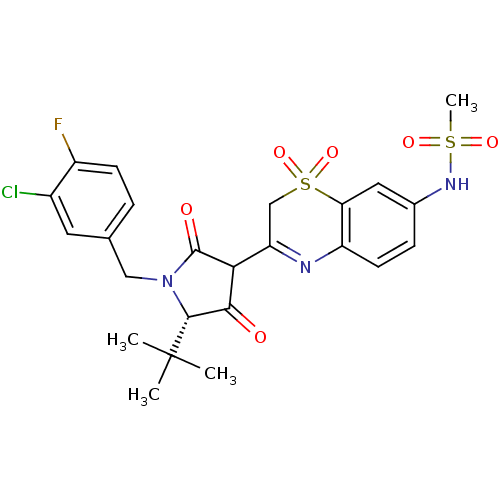 (CHEMBL570934 | N-{3-[(S)-5-tert-Butyl-1-(3-chloro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300496 (CHEMBL577404 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300505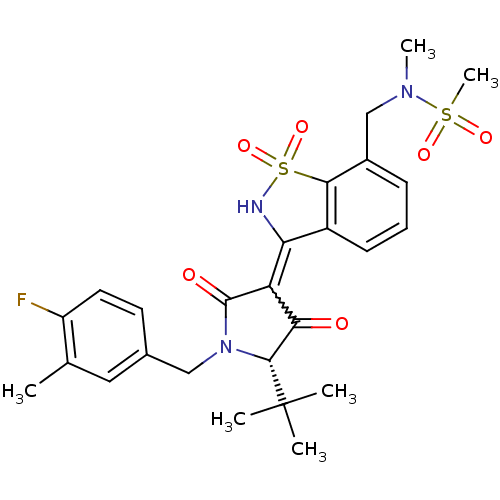 (CHEMBL572683 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301906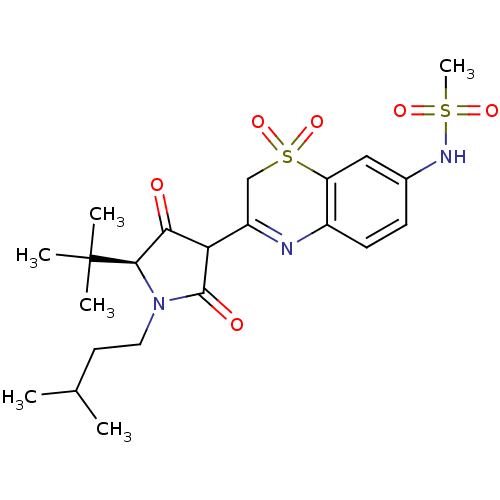 (CHEMBL571606 | N-{3-[(S)-5-tert-Butyl-4-hydroxy-1-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307539 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307540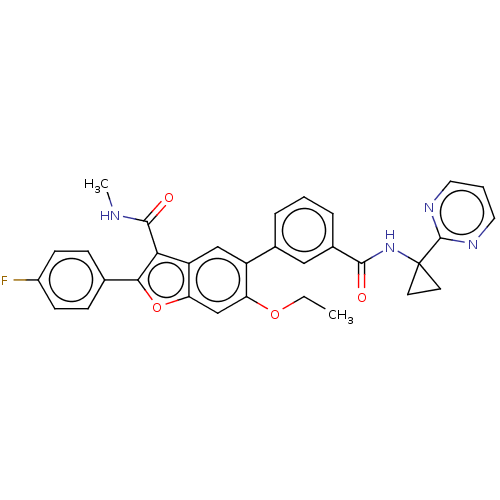 (6-Ethoxy-2-(4-fluorophenyl)-N-methyl-5-(3-((1-(pyr...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.78 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301913 (CHEMBL571564 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300501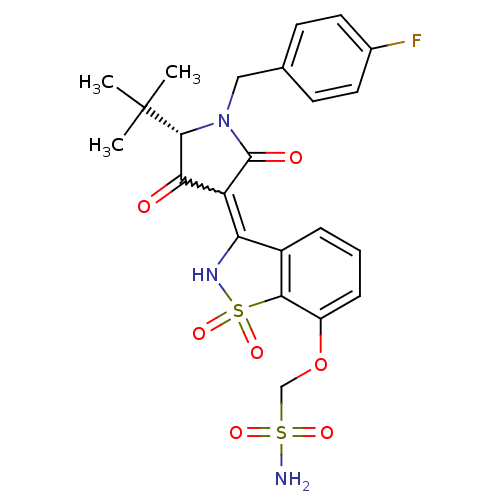 (CHEMBL573175 | {3-[(S)-5-tert-Butyl-1-(4-fluoro-be...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 972 total ) | Next | Last >> |